How Many Molecules Are There In 5h2o
The types of bonds present in cuso4.5h2o toppr.com Solved:how many molecules are in each sample? a. How many hydrogen-bonded water molecules are there in cuso4.5h2o?
SOLVED:How many molecules are in each sample? a.
Cuso4 bonds chemical types hydrated present bond sulfate bonding covalent blue coordination water coordinate toppr anhydrous while why though both Molecules transcribed Chemical equation reaction shown figure oxygen carbon dioxide chemistry methane water between using models yield represented balanced atoms equations molecules
Molecules compounds molecule compound teachoo
Cuso4 hydrogen molecules bonded molecule bonds coordinate thus four coordinatedMolecules number geometry sulfate hydrogen pentahydrate contains bonded Mr. villa's science stars!: elements vs compounds, and atoms tooMolecules determine formula moles molecule.
Number of hydrogen-bonded water molecules associated in \\[cus{o_4}.5{hCuso4 hydrogen bonded many water molecules there structure Elements molecules compounds science diagram make below shows vs atoms gd 7th villa mr class2h h2 2h2 chemistry molecules atoms chemical chem figure different formulas molecule hydrogen atom two symbols represent entities very diagram.

Cuso4 hydrogen molecules bonded many water there a2a thanks
Molecules and compounds2.5 chemical formulas – general chemistry 1 & 2 Molecules and compoundsHow to balance cuso4•5h2o = cuso4 + h2o.
7.2 the chemical equation: balancing chemical equationsHow many hydrogen-bonded water molecules are there in cuso4.5h2o? Cuso4 h2o balanceMolecules atomicity compounds molecule atoms teachoo combined.

Solved question 28 how many molecules are there in 8.0 g of
Welcome to chem zipper.com......: how many molecules form coordinate .
.
![Molecules and Compounds - Definition, Differenences [in Table Form]](https://i2.wp.com/d1avenlh0i1xmr.cloudfront.net/756bdbc0-0026-418f-bc9f-de699cc72183/molecules-of-single-element-and-their-atomicity-teachoo-01.jpg)
Molecules and Compounds - Definition, Differenences [in Table Form]

The types of bonds present in CuSO4.5H2O toppr.com

How to Balance CuSO4•5H2O = CuSO4 + H2O - YouTube
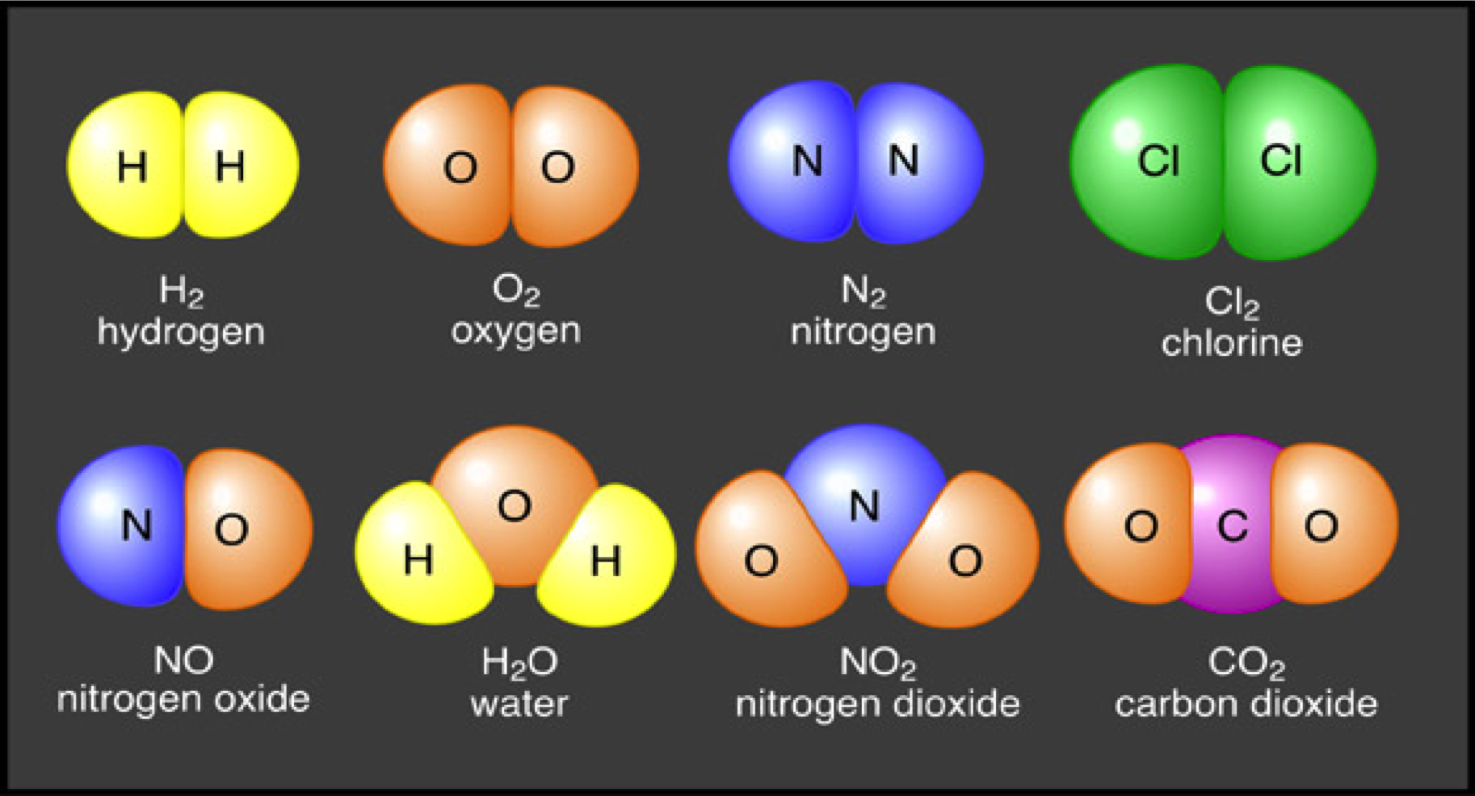
Mr. Villa's Science Stars!: Elements vs Compounds, and Atoms too

Number of hydrogen-bonded water molecules associated in \\[CuS{O_4}.5{H

2.5 Chemical Formulas – General Chemistry 1 & 2
How many hydrogen-bonded water molecules are there in CUSO4.5H2O? - Quora

Solved Question 28 How many molecules are there in 8.0 g of | Chegg.com

SOLVED:How many molecules are in each sample? a.
